Our Products
- REQUEST A -
QUOTE
- SCHEDULE A -
DEMO
SLIMStat
SLIMStat is extremely easy-to-use software developed and validated specifically to determine the shelf life of drug products that have been placed on stability. This interactive and intuitive tool empowers non-statisticians the ability to generate statistical analyses with complete confidence. Fully validated and 21 CFR Part 11 compliant, SLIMStat accepts data directly from the SLIM database, keyboard, clipboard, or any suitably-formatted text/Excel file. The product’s shelf life for single or pooled studies are then automatically calculated. It’s that easy!
SLIMStat also features a GAMP 5 risk-based validation methodology that allows for a stream-lined-yet-comprehensive, ready-to-execute IQ/OQ validation package – no need for complex testing of endless configurations.
Key Features and Benefits
- Determines shelf life using one-sided or two-sided 95% confidence intervals
- Quickly and easily calculates shelf life, trend analysis, out-of-trend (OOT) determinations (within a single batch or across multiple batches), ICH Q1E batch pooling, back-purity, and kinetic analyses (Arrhenius Equation)
- Graphical visualization of the data can be saved as Windows Metafiles or PDFs for easy insertion into other documents
- A company logo (bitmap) may be added to the header of a SLIMStat printout
- Generates reports in English, French, German, Japanese, Polish, Portuguese, and Spanish languages
- Allows your company to implement ICH Guidance for Industry “Q1E Evaluation of Stability Date,” including “Pooled Mean Squared Error” and “Analysis of Covariance” testing for poolability of batches
- ANACOVA calculations yield the same industry recognized results as the program used by the FDA
- Configure reports for international acceptance with user configurable decimal separator and date formats, UNICODE compliance, and display of non-localized date-time audit logs
- User-selected treatment of Limit-of-Detection values
- A tool to save R&D formulators time and money utilizing kinetic analysis (Arrhenius Calculation)
- Reduces regulatory risk by use of a program that is fully validated according to GMP guidelines and can be implemented as 21 CFR Part 11 compliant
- Empowers your chemists and pharmacists, and formulators with an extremely easy to use tool to make good decisions based on sound statistical principles
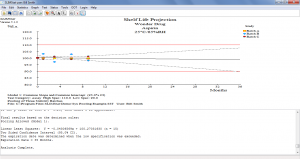
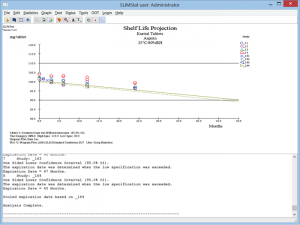
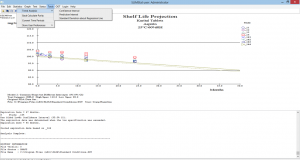
Long Term/Standard Conditions & Kinetic Analysis Calculations
- SLIMStat determines shelf life using one-sided or two-sided 95% confidence intervals
- ANACOVA calculations are used for determining the validity of pooling multiple studies
- The studies are assigned a Model I, II, or III/IV designation consistent with ICH Q1E guidelines
- Automatically makes decisions as to the validity of pooling batches/studies. No guesswork!
- Tentative expiration dating periods may be estimated by processing accelerated stability data using the Arrhenius Equation
- The shelf life may be predicted at any valid temperature with the click of a button
Click the thumbnail to see a larger image of the Pooled Batches screen
Click the thumbnail to see a larger image of the Standard Storage Conditions Model Analysis
Click the thumbnail to see a larger image of the Accelerated Storage Conditions Analysis
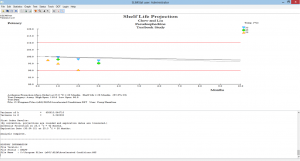
Trend Analysis
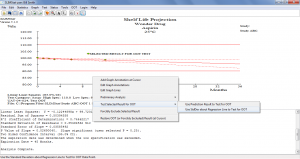
Out-Of-Trend (OOT) Determinations
- Simply select a result to examine and SLIMStat can immediately determine if it is statistically OOT or not and will notate accordingly
- OOT determinations can be made for a data point within a single batch or for a data point across many batches
- Choice of using Prediction Interval or the Standard Deviation about the Regression Line (using either the calculated Std Dev or can input the known Std Dev of the Population)
Click the thumbnail to see a larger image of the OOT Determination screen
Back Calculation Of Purity
- The user can enter a target time (in units of hours, days, weeks, or months, depending on the time units of the displayed analysis) to calculate the initial purity of the drug required to obtain the desired projection or expiration date.
- User can select “Projection” type of calculation (based solely on Y=mx+b) or “Expiration Date” type of calculation (based on the displayed Confidence Interval).
21 CFR Part 11 Compliance
SLIMStat’s functionality meets the Electronic Records and Electronic Signatures rules of 21 CFR Part 11 as they relate to “closed” systems. It features multiple levels of security, including prompted password expiry, a time-out procedure, the ability for each SLIMStat user to have different security rights, and a full audit trail.
H&A Scientific’s SLIM. By stability professionals. For stability professionals.
Click Here to Request Information or schedule a WebEx Demonstration