Our Products
- REQUEST A -
QUOTE
- SCHEDULE A -
DEMO
PC/Chrom
H&A Scientific’s PC/Chrom is the complete chromatography workstation solution your company needs. In one simple package, it interfaces your company’s HPLC, GC, or CE detector with a personal computer or corporate network server.
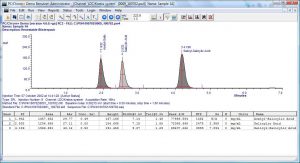
PC/Chrom reads the analog output signal from a detector, digitizes the signal, displays the chromatogram, and processes data to produce a final, printable report — all according to user specifications.
Like all H&A Scientific products, PC/Chrom is a turnkey solution. It includes all necessary software, which is FDA-regulations and GAMP-guideline validated, and hardware, such as power supply and detector wiring. The complete PC/Chrom configuration features:
Hardware
- The PC/Chrom Analog-to-Digital (A/D) converter(s) communicate with your PC to acquire, process, and report the analysis.
- Two versions of the A/D Hardware are available: 1) An Ethernet (TCP/IP) interface, or 2) A Serial Port (RS232/USB) interface.
- 1 to 4 independent time-based channels (A/D converters) can be operated per single computer.
- Each acquisition channel (A/D converter) uses a high-resolution 24-bit Delta-Sigma A/D converter.
- The Ethernet Hardware has an analog input range of +/- 2000 mV. The Serial Port Hardware has an analog input range of +/- 1250 mV.
- The Ethernet Hardware supports data acquisition rates of 4, 8, 16, and 32 samples per second (Hz). The Serial Port Hardware supports data acquisition rates of 1, 2, 4, 8, and 16 samples per second (Hz).
- Support for valve contact closure or TTL start signal, or data acquisition may be initiated from the keyboard.
- Support for up to four (4) TTL Output events, e.g., autozero of detector.
- Each A/D converter is enclosed in external sturdy metal case.
Software
- Fully validated according to FDA Regulations, GAMP Guidelines, and our Quality System for use with Windows 2000, XP, Vista, and Windows 7.
- Electronic Records features per FDA 21 CFR Part 11, including full audit trails (event logs) and password protection.
- Data collection may be initiated within a few seconds upon running PC/Chrom, either using default or recalling stored parameters.
- PC/Chrom creates unique folders for each chromatographic run, thus simplifying disk organization.
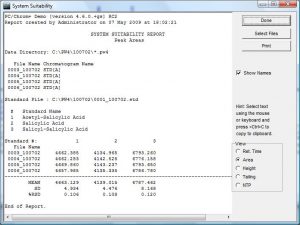
- Automatic peak integration and calculation of unknown sample concentrations, using either external or internal standards. Manual peak integration is also possible.
- Response factors may be entered to specify calibrations based on standards that are not present in the standard solution.
- Uncalibrated peaks may be quantitated using a specified standard.
- Chromatographic overlays are supported.
- Capillary Electrophoresis (CE) specific calculations are also supported.
- A Certificate of Validation, User Manuals, and Installation Qualification (IQ) documents are included with all orders.
- Operational Qualification (OQ) and Performance Qualification (PQ) services are additional options that are available. Please contact us for information about these services.
Click here to Contact Us or Request Information
Click any of the image below to see a larger view